News
-
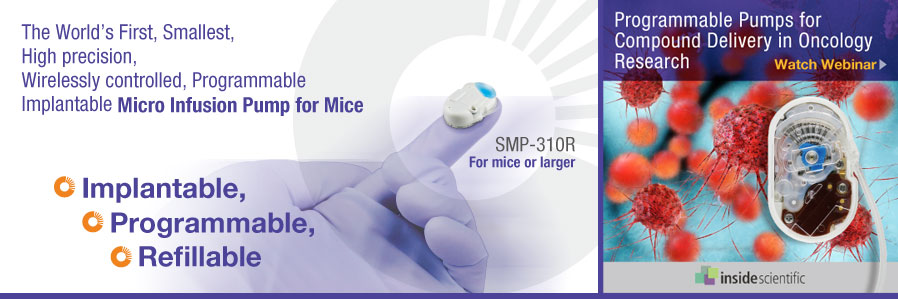
 Have you ever wanted to get more out of your in-vivo pharmaceutical studies? Have a burning question about programmable pumps and implication of refinement and reduction of animal use? Join Dr. Christian Schnell in his upcoming webinar on Programmable pumps for compounds delivery in oncology research: implication for refinement and reduction of animal use. More information and registration < here >
May 17, 2021
Have you ever wanted to get more out of your in-vivo pharmaceutical studies? Have a burning question about programmable pumps and implication of refinement and reduction of animal use? Join Dr. Christian Schnell in his upcoming webinar on Programmable pumps for compounds delivery in oncology research: implication for refinement and reduction of animal use. More information and registration < here >
May 17, 2021
-
 iPRECIO Programmable Minipumps for continuous delivery lenalidomide (LLD) in chronic lymphocytic leukemia (CLL). Video, https://youtu.be/d8CHR7et5zs from Start to 7 minutes: Rodent Studies of PK, Safety and Tolerability of LLD in Healthy and SCID Mice by Dr Jamie Oliver Chief Medical Officer of Starton Therapeutics (https://startontx.com/). IMS/SMP-300 for mice or larger used. Photo of SMP-200 for rats of larger shown.
February 24, 2021
iPRECIO Programmable Minipumps for continuous delivery lenalidomide (LLD) in chronic lymphocytic leukemia (CLL). Video, https://youtu.be/d8CHR7et5zs from Start to 7 minutes: Rodent Studies of PK, Safety and Tolerability of LLD in Healthy and SCID Mice by Dr Jamie Oliver Chief Medical Officer of Starton Therapeutics (https://startontx.com/). IMS/SMP-300 for mice or larger used. Photo of SMP-200 for rats of larger shown.
February 24, 2021
-
 New review article on iPRECIO® - Linkedin Article – “Programmable pumps offer precise and translatable delivery of compounds to stress-free animals”
New review article on iPRECIO® - Linkedin Article – “Programmable pumps offer precise and translatable delivery of compounds to stress-free animals”
https://www.linkedin.com/pulse/programmable-pumps-offer-precise-translatable-delivery-tsung-tan/?trackingId=i9CxNGMbQJye6JF6HVLJSw%3D%3DFebruary 9, 2021
-
 US20200330445A1: Continuous delivery of lenalidomide and other immunomodulatory agents
US20200330445A1: Continuous delivery of lenalidomide and other immunomodulatory agents
"After the tumor reached an average size of 100-150 mm, iPrecio pump was surgically implanted into each of the mice . Dosing began twenty - four hours post pump implantation. Each of Groups 3-6 was treated lenalidomide via continuous subcutaneous infusion at different hourly rate. The dosing lasted 14 days followed by one day off the treatment and lasted for another 14 days . The iPrecio pump was replaced after 14 days." For full reference: - Click -December 1, 2020
-
 IMS-200 version 13 Revision 532renew has been released
IMS-200 version 13 Revision 532renew has been released
An important Application Software update has been released. Please contact us for downloading and installation instructions. Please share with us UCD-200 Serial Number. It can be found on the back of the programming device which plugs into the USB port of your PC. The upgrade is free and compatible with windows 10. Contact us <click here>September 18, 2020